/ News, Research
Human BLB on-a-chip to bridge the gap between preclinical and clinical studies (Bodmer Lab)


Innosuisse offers a comprehensive toolbox of assistance, supporting innovation across all stages, from early lab discoveries to launching successful companies. In collaboration with the SNSF, it plays a pivotal role in driving the growth of Swiss startups and innovation, providing exciting funding opportunities also for DBM researchers. One such team, led by PD Dr. Vesna Petkovic and including N. Chatelain, A. Korah, R. Trajanoska, and M. Sekulic, as part of the Bodmer inner ear research group, received substantial support over the years from Innosuisse, as well as FAG from the Animal-free Research Foundation for their groundbreaking development of the human blood-labyrinth barrier (BLB) organ-on-chip system.
Hearing loss is one of the leading global causes of disability. Despite this, effective pharmacological treatments to enhance hearing have yet to reach the market. Inner ear auditory hair cells and the blood-labyrinth barrier play a crucial role in maintaining normal hearing. The BLB, located between the systemic circulation and stria vascularis, is essential for cochlear and vestibular homeostasis. It facilitates nutrient and metabolite transport into the cochlea, and protects the cochlea from inflammation and disease. Delivering therapeutics to the cochlea and vestibular system of the inner ear is complicated due to their inaccessible location. It is particularly difficult to avoid adverse effects due to off-target binding, such as ototoxicity from antibiotics and chemotherapy.
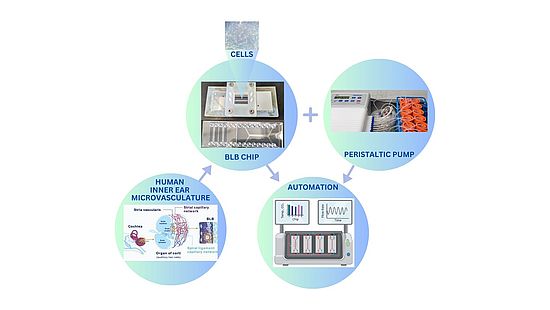
To address the complexity and inaccessibility of the BLB, we have developed the first micro-engineered, three-dimensional model of a BLB cell culture on a chip, using progenitor cells from autopsy-derived adult human temporal bones. This chip serves as a tool for investigating the specific contributions of the BLB to physiological and pathophysiological mechanisms underlying hearing loss. The innovative system is the first of its kind designed specifically for studying the BLB in the inner ear. In collaboration with CSEM, a leading Swiss technology and innovation center, the group is also working on is developing an automated chip handling system, with the first prototype version is currently in development.
The main advantages of the BLB chip system include the use of human cells derived directly from the fine vessels of the inner ear, closely replicating physiologically relevant aspects at the cellular level. This allows for the testing of various barrier properties following exposure to test compounds. Currently, aspects that can be monitored include barrier integrity through TEER measurements, barrier permeability using standard FITC-Dextran protocols, and direct assessment of compound passage with concentration measurement via HPLC over time. Additional protocols enable monitoring of cell toxicity through LDH tests, immunocytochemistry staining, imaging for target proteins, and transporter assays. Initial tests involving compounds in development have already been conducted on the BLB system, utilizing some of the compounds supplied by Topadur Pharma AG.
The BLB chip stands out due to its distinctive features, including biocompatibility, animal-free composition, sustainability, and scalability. It can mimic disease tissues, such as those found in Meniere's Disease or inflammatory conditions. The chip is versatile and can be adapted to different cell types, making it a promising tool for drug development, toxicity testing, and transport assays. This advancement opens up new possibilities for drug discovery in treating inner ear disorders, potentially leading to significant improvements in patient lives. The added value of this innovation can significantly impact the study of the inner ear, making drug discovery more cost-effective and time-efficient, and enhancing the predictive value of potential preventive and curative oral treatments. International patent rights have been secured for the BLB chip, and the patent for automation is currently underway. The DBM congratulates the team on this remarkable achievement.