/ News, Research
Durable Lymphocyte Subset Elimination to Decipher Immune Control of Chronic Viral Infection (Pinschewer lab)


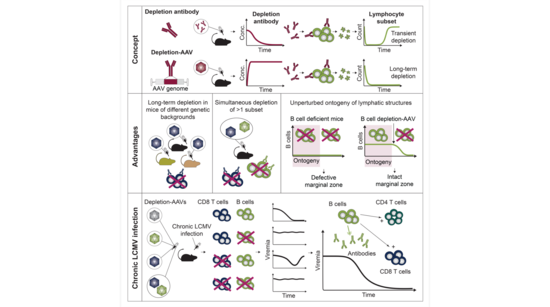
In her recently published work, Anna Lena Kastner and colleagues have developed a novel tool to durably eliminate lymphocyte subsets in mice, and have used this tool to decipher how antibody-producing B cells and cytotoxic T cells cooperate in controlling chronic viral infection.
Monoclonal depletion antibodies (depletion-mAbs) and genetically engineered mouse models are commonly used to interrogate the roles of specific immune cells in infection, cancer, and autoimmunity. In this study, the authors sought to develop a tool that combines the advantages of these methods while avoiding key limitations.
They engineered adeno-associated viral vectors (AAVs) that express depletion-mAbs (depletion-AAVs), enabling the long-term elimination of lymphocyte subsets after a single administration to mice. Depletion-AAVs can simultaneously deplete multiple lymphocyte subsets, they are effective across different genetic backgrounds, and allow normal development of lymphatic structures—avoiding deficiencies in genetically engineered mouse models such as marginal zone defects in B cell-deficient animals.
Equipped with this innovative tool, the researchers investigated the contributions of B cells and CD8+ T cells to resolving chronic viral infections. They demonstrated that B cells are essential for unimpaired CD4+ and CD8+T cell responses to lymphocytic choriomeningitis virus in mice. Most notably, CD8+ T cells failed to suppress viremia when B cells were depleted long-term, showing that the resolution of chronic infection by CD8+ T cells depends on antibodies reducing viral loads.
This study introduces depletion-AAVs as a versatile tool for immunological research and unveils the intricate interdependence of and synergy between B cells and CD8+ T cells in chronic viral infection.
The DBM congratulates the Pinschewer Lab on this accomplishment.