/ News, Research
New insights in the understanding of a highly aggressive form of childhood leukemia (Schwaller Lab)
New research has shed light on the molecular mechanisms behind a rare and aggressive form of childhood leukemia known as pure red cell leukemia (PEL). The study, published in the journal Blood, was led by Juerg Schwaller and his team at the DBM.
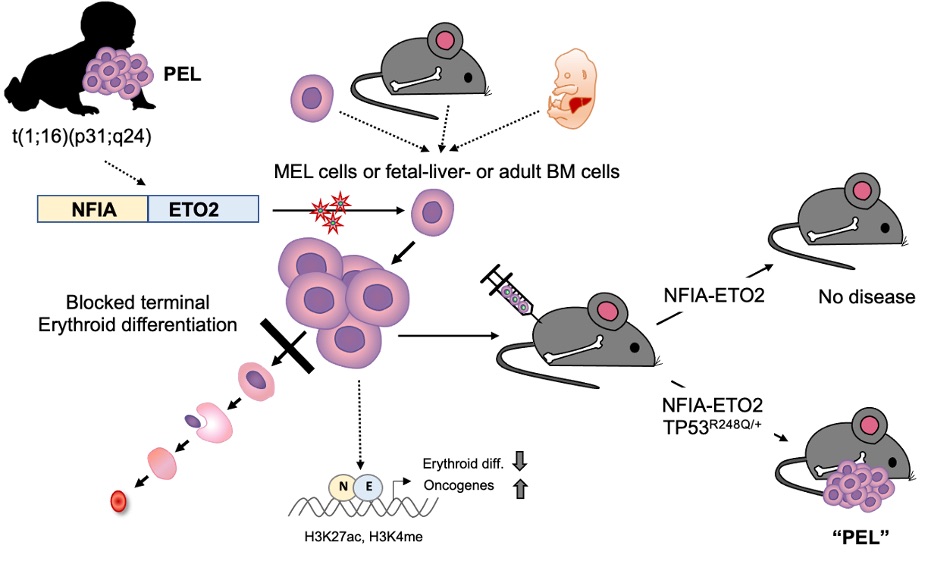
PEL is a rare hematological malignancy characterized by chromosomal translocations that lead to the expression of fusion genes, such as NFIA-ETO2. However, the molecular pathogenesis of PEL remains mostly unknown, making it challenging to develop effective treatments.
In this study, the researchers investigated the role of NFIA-ETO2 in the pathogenesis of PEL by expressing the fusion gene in primary fetal and adult mouse erythroblasts. They found that the fusion promotes proliferation and inhibits terminal differentiation of murine erythroblasts in vitro. However, it was not sufficient to cause disease when transplanted into irradiated recipient mice. The researchers observed that when NFIA-ETO2 is expressed in the presence of a PEL-associated TP53R248Q mutation, it leads to aberrant clonogenic potential and PEL disease upon transplantation.
Furthermore, the team used combined transcriptome and chromatin-conformation analyses to show that transformation by NFIA-ETO2 is dependent on NFIA-DNA binding and the ETO2 NHR1-4 domains. This causes the fusion gene to act as an abnormal transcription factor that primarily binds to and represses erythroid genes containing NFI binding sites and/or are decorated by ETO2, resulting in a shift in activity from GATA- to ETS-motif-containing target genes. TP53R248Q on the other hand does not affect erythroid differentiation, but provides self-renewal and survival potential.
These findings, provide important insights into the pathogenesis of PEL, which may pave the way for future therapeutic interventions. However, Juerg Schwaller cautioned that while NFIA-ETO2 experimentally cooperates with TP53R248Q, TP53 mutations are rare in pediatric patients. This highlights the need for further research to substantiate alternative mechanisms such as mutations in KIT, ARID1A and MUTYH, which are also observed in NFIA-ETO2+PEL cells.
Social Media
