Metabolic disease . Diabetes . Obesity . Metabolism . Immunology . Macrophages
Translational Diabetes
Gut mucosal immunity in metabolic disease
Diabetes is a serious and growing health problem, affecting 463 million people worldwide in 2019. Chronic inflammation is a hallmark in the pathogenesis of type 2 diabetes and obesity. However, the initiating mechanisms of glucose dysregulation and inflammation in obesity remain poorly understood.
Our research focuses on gut mucosal immunity as a potential starting point of inflammation and glucose dysregulation in metabolic disease. Thereby, we are interested in elucidating the role of the mucosal immune system, especially intestinal macrophages. Intestinal macrophages constitute the largest macrophage compartment within the body and could serve as crucial “sensors” of environmental or external factors (i. e., food intake or environmental toxins such as air pollutants or cigarette smoke particles) for the host’s immune system. We are interested in how intestinal macrophages respond to such environmental stressors and how their response ultimately affects glucose homeostasis and systemic inflammation.
We found that pro-inflammatory/ monocyte-derived colonic macrophages are elevated in mice fed a high fat diet, suggesting a link between colon macrophage numbers and glucose homeostasis. Indeed, we were able to establish a direct link between colon macrophages and glucose metabolism as colon-specific macrophage depletion ameliorated glycemic control and beta-cell function.
Besides classical risk factors like unhealthy food or sedentary lifestyle, also environmental toxins like air pollution or cigarette smoke increase the risk for type 2 diabetes. We showed that air pollution particles mediate diabetes via the gastrointestinal tract rather than via lung exposure as previously thought. Oral exposure to pollutants occurs via mucociliary clearance of particles from the airways with subsequent swallowing of the particles. Particles reaching the gastrointestinal tract caused a distinct inflammatory shift of colon macrophages. This local gut inflammation was associated with impaired insulin secretion and glucose intolerance. Similarly, we are assessing how cigarette smoke particles and e-liquids from e-cigarettes impact on mucosal gut immunity and glucose homeostasis. Also, we are studying the differential effects of air pollution particles on atherosclerosis when they are administered via lung or gut exposure.
In addition, we are also interested in therapeutic aspects of gut mucosal immunity. As weight- loss surgeries are associated with a rapid improvement in glucose homeostasis, we aim to address the question whether weight loss has beneficial effects on gut mucosal immunity and hence glucose tolerance.
In sum, the aim of our laboratory is to better understand the changes of gut mucosal immunity upon environmental stimuli and how this connects with glucose intolerance and systemic inflammation in metabolic disease to potentially find immune-modulatory treatments.
Connection to Clinical Practice
Prof. Dr. Petr Hruz, Prof. Dr. Jan Niess1
Prof. Dr. Ralph Peterli2
PD Dr. Bettina Wöllnerhansen2
PD D. Anne Christin Meyer-Gerspach2
PD Dr. Tarik Delko2
1 Clarunis, University Center for Gastrointestinal and Liver Diseases Basel, Basel, Switzerland
2 Department of Visceral Surgery, University Hospital Basel, Basel, Switzerland.
Human intestinal macrophages in metabolic disease
In order to translate our findings to human disease, we compare human gut biopsies of patients with different environmental “toxins” (i.e., normal weight versus overweight individuals or smokers versus non-smokers). We found an inflammatory shift in intestinal macrophages in obese individuals, similar to our findings in obese mice. To assess whether these changes in gut innate immunity are reversible, we plan to study intestinal macrophages from individuals before and after weight loss surgery. The ultimate goal is to potentially find immunemodulatory treatments at the interface between the environment and the host’s metabolism.
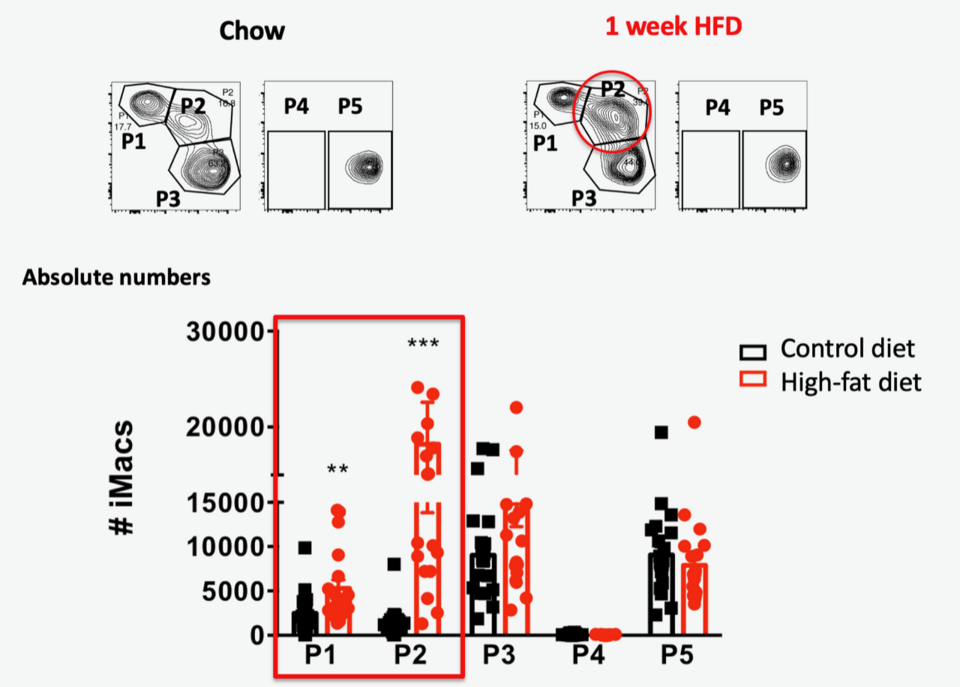
Fig. 1: Pro-inflammatory colon macrophages are elevated following high-fat diet. Top panel: Representative flow cytometry of colon macrophage subpopulations P1-P5 subpopulations in a mouse fed standard chow or 1 week high-fat diet. Bottom panel: Absolute numbers (#) of pro-inflammatory (P1, P2, P3) and anti-inflammatory/resident colon macrophage subpopulations (P4, P5) in mice fed standard chow or high-fat diet.
