Precision oncology . Cancer Biomarkers . Visceral Cancers . Patient-Derived . Organoids Drug Testing . Multi-Omics Profiling
Visceral Surgery and Precision Medicine
Integrative approaches to augment precision medicine in cancer
Precision medicine is about getting the right treatment to the patients at the right time. Taking rectal cancer as an example, patients with intermediate-stage rectal cancer are typically treated with chemoradiotherapy followed by surgical excision to remove any residual disease. Post-surgery histologic analysis reveals that ~25% of the patients achieve complete response to chemoradiotherapy, meaning that they would not have needed surgical intervention. For the remaining patients that do not achieve complete response to chemoradiotherapy, alternative therapeutic options are desirable. Thus the identification of robust biomarkers that will precisely predict which patients would likely achieve good response to chemoradiotherapy, thus sparing them the unnecessary surgery, are urgently needed. On the other hand, model systems that reflect the biological behavior of the tumors in individual patients to test alternative therapeutic options in an ex vivo manner are essential. Our lab takes several complementary approaches to address outstanding challenges in precision oncology.
Ex vivo models for drug testing
Patient-derived organoids (PDOs) have been shown to retain the molecular features of the original tumors and to better resemble tumor heterogeneity than traditional two-dimensional cell culture methods derived from single cell clones. They are thus frequently used as ex vivo preclinical models for drug response prediction. In collaboration with the visceral surgeons at the Clarunis, we have initiated the prospective collection of patient tumors to establish a living biobank of PDOs. To date, we have successfully established a collection of ~100 PDOs (Fig. 1a). We have also established a protocol to test drugs and monitor response in real-time over five days. Using this protocol, we showed that 6-mercaptopurine effectively reduces cell growth and induces apoptosis in colorectal tumors with overexpression of ADSL, a gene with a critical role in purine synthesis (Fig. 1b).
Discovery of cancer vulnerabilities
The concept of synthetic lethality has helped extend precision oncology to enable the targeting of undruggable genes by disrupting their genetic interactors. For instance, GATA3 is mutated in 15-18% of estrogen-receptor positive breast cancer but is not targetable. By systematically interrogating a large-scale RNAi screen, we identified MDM2 as a top synthetic lethal interactor of GATA3. We found that pharmacological inhibition of MDM2 with idasanutlin reduces cell proliferation and induces apoptosis in GATA3-deficient cancers in vitro and in two independent in vivo models (zebrafish and chicken chorioallantoic membrane models). GATA3-mutant PDOs are also more sensitive to idasanutlin than GATA3-wild-type PDOs (Fig. 2a-b). With idasanutlin and other MDM2 inhibitors widely available, our findings can be rapidly translated into clinical trials to evaluate in-patient efficacy and GATA3 mutation status as a predictive biomarker of response to idasanutlin in breast cancer.
Cell-free DNA as a minimally invasive tumor surrogate
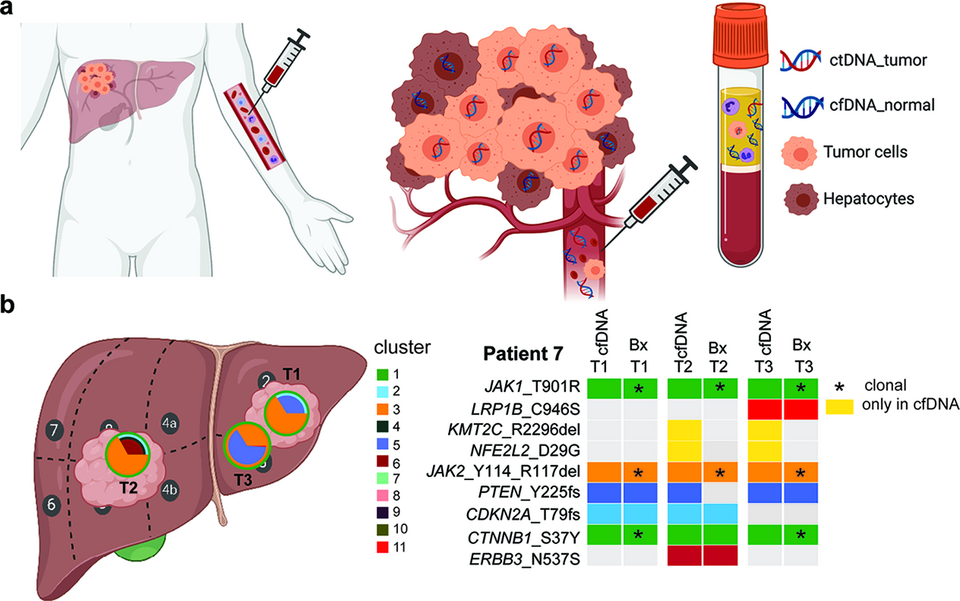
Precision medicine is only possible with tumor materials, and those that can be obtained through minimally invasive procedures (e.g. blood draw) are particularly desirable. Plasma-derived cell-free DNA (cfDNA) has been shown to reflect the genetic makeup of tumors and may serve as a tumor surrogate for mutation detection and disease monitoring during the course of therapy. cfDNA may be particularly useful as a genetic surrogate for tumors difficult to or not amenable to biopsy. This is important for hepatocellular carcinoma (HCC) as its diagnosis does not always require tissue biopsy; tumor tissue materials in inoperable patients are usually unavailable. The lack of tumor materials hinders the wider adoption of precision medicine in HCC. We found that genetic profiling of plasma-derived cfDNA was an adequate surrogate of primary HCC in patients with large tumor or metastatic disease (publication awarded the Swiss Foundation against Liver Cancer 2019 award). We are now investigating whether cfDNA may help disease monitoring in HCC (Fig. 3).
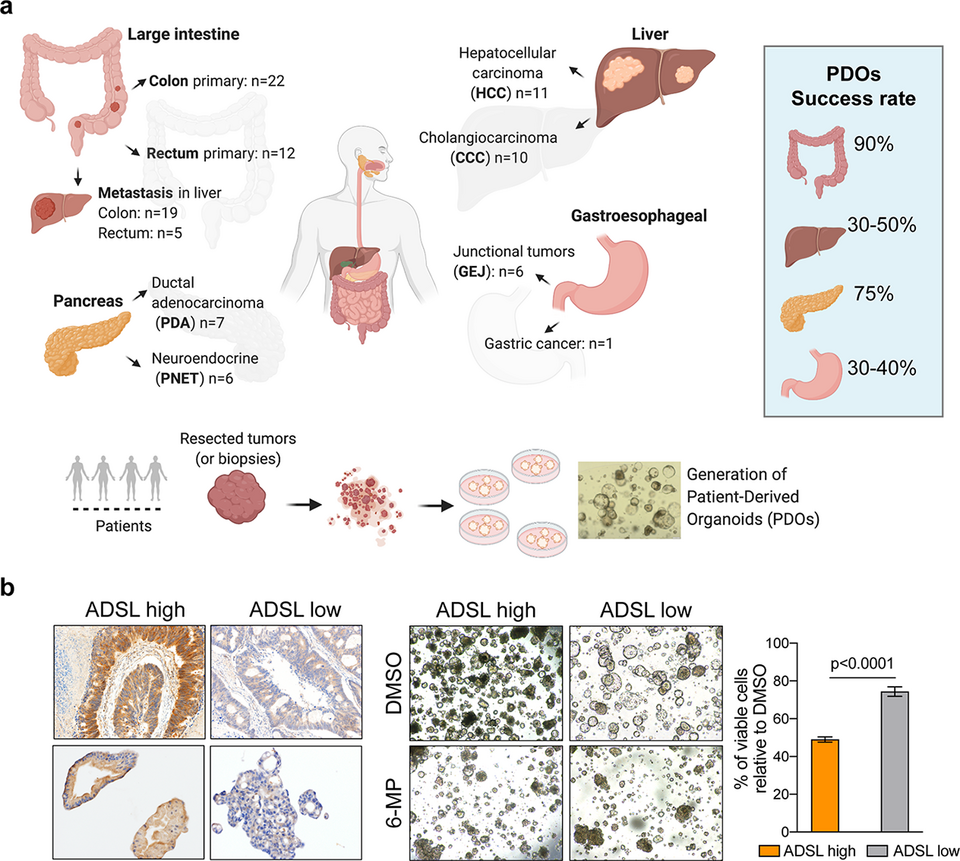
Fig. 1: (a) We have optimized protocols to derive patient-derived organoids from tissue specimens. In collaboration with the surgeons at Clarunis, we have established ~100 organoids in our living biobank. (b) Our drug screening protocol in PDOs enabled us to demonstrate that ADSL overexpression is a biomarker of response to 6-mercaptopurine in colorectal tumors.
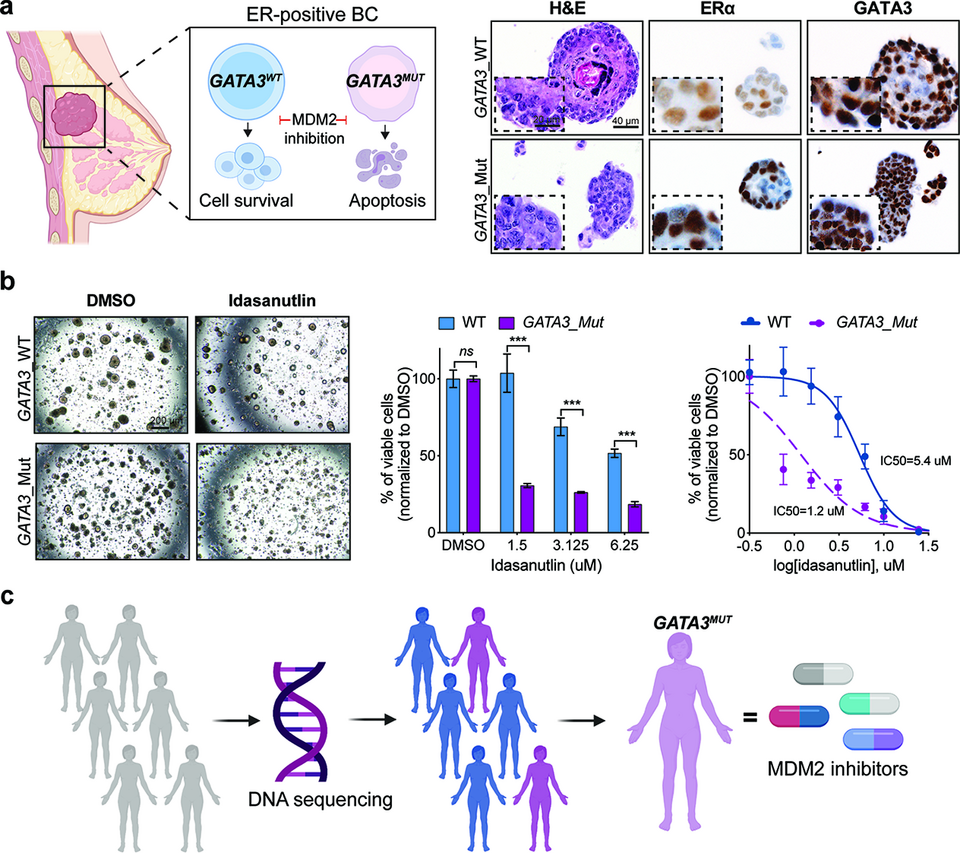
Fig. 2: (a) MDM2 is a novel synthetic lethal target in GATA3-mutant estrogen receptor (ER)-positive breast cancer. Organoids derived from GATA3-wild-type (WT) and GATA3-mutant breast cancers. (b) GATA3 mutations sensitize PDOs to idasanutlin treatment. (c) Genetic screening strategy to enable the precise targeting of the GATA3-mutant breast cancer.