GABA-B . GABBR2 . Amyloid Precursor Protein . APP . Rett Syndrome
Epileptic Encephalopathy
Molecular Neurobiology Synaptic Plasticity
From the GABA-B receptor proteome to brain functions
and therapeutic concepts
My laboratory studies how the molecular composition of GABA-B receptors
(GBRs), the G protein-coupled receptors for the neurotransmitter GABA, influences
neuronal activity. Because GBRs are implicated in the pathophysiology of neurological
and psychiatric disorders, we also aim at targeting molecularly defined
GBR signaling complexes for therapy. In collaboration with Prof. B. Fakler (University
of Freiburg, Germany), we identifed 30 GBR-associated proteins (Pin & Bettler,
Nature 540, 2016; Bettler & Fakler, Curr. Opin. Neurobiol. 45, 2017). We have
mapped the interactions of several of these proteins with each other and with the
GBR subunits GB1 and GB2 (Fig. 1). We found that GBR components associate
in a modular fashion into a variety of functionally distinct multi-protein complexes.
We analyzed several GBR-associated proteins for their effects on receptor signaling,
neuronal excitability, brain network activity and behavior. Auxiliary KCTD proteins,
for example, regulate the kinetics of GBR-induced currents, explaining kinetic
discrepancies between currents observed in different neurons (Fritzius et al., J.
Neurosci. 37, 2017). KCTD proteins influence both strength and frequency of thalamic
spindle oscillations, showing that kinetic effects of the KCTDs on GBR signaling
regulate network activity (Ulrich et al., Neuropharmacol. 136, 2018). Accordingly,
lack of KCTD16 in mice also influences behavioral responses (Cathomas et
al., Behav. Brain Res. 317, 2017).
Amyloid precursor protein (APP), adherens junction-associated protein 1 (AJAP1)
and PILRα-associated neural protein (PIANP) form three mutually exclusive GBR
complexes by binding to the N-terminal sushi domain of GB1a (Schwenk et al., Nat.
Neurosci. 19, 2016). Because this sushi domain mediates axonal localization, we
tested whether axonal trafficking of GBRs is impaired in APP-/-, AJAP1-/- or PIANP-/-
mice (Dinamarca et al., Nat. Commun. 10, 2019). Selectively APP-/- mice
exhibited a decrease in axonal GBRs (Fig. 2A) and a consequent deficit in GBRmediated
inhibition of neurotransmitter release. Trafficking of APP/GBR complexes
in axons was visualized using time-lapse imaging (Fig. 2B-D). APP associates
with JIP3 and CSTN3 proteins (Fig. 1) of the axonal trafficking machinery. Complex
formation with GBRs stabilizes APP at the cell surface and reduces proteolysis of
APP to Aβ, a component of senile plaques in Alzheimer’s disease. These findings
establish a link between APP/GBR complex formation, axonal trafficking of GBRs
and Aβ production.
The SHRM4 protein, which is genetically associated with intellectual disability and
epilepsy, controls GBR cell surface expression. Knockdown of Shrm4 in rodents
impairs GBR activity, induces anxiety-like behaviors and increases susceptibility to
seizures (Zapata et al., Nat. Commun. 8, 2017). Collaborative work further showed
that GBRs shape the auditory map (Vickers et al., Neuron 99, 2018), evoke distinct
responses in astrocytes (Mariotti et al., Nat. Commun. 9, 2018) and regulate cocaine-
induced behaviors (Edwards et al., Nat. Neurosci 20, 2017).
Drug Discovery
Clinical use of GBR drugs is limited to agonists and the treatment of narcolepsy,
spasticity and alcohol use disorders. One reason for the limited use of GBR agonists
is that global GBR activation results in unwanted side effects. The recognition
that GBRs form a variety of multi-protein complexes provides opportunities for targeting
cell-type specific functions and individual signaling pathways (Rosenbaum
et al., Nat Rev Drug Discov (2020). https://doi.org/10.1038/s41573-020-0086-4.
Targeting molecularly defined receptor complexes should reduce side effects and
enable new therapeutic applications. In collaboration with the medicinal chemistry
group of K. Strømgaard (Pharmaceutical University, Copenhagen), we developed peptide-based inhibitors of the GBR/KCTD interaction (Sereikaite et al., J. Med.
Chem. 62, 2019). These inhibitors are expected to exhibit anxiolytic properties
by preventing GBR desensitization. We are currently also developing peptides interfering
with defined presynaptic GBR complexes to facilitate glutamate release,
particularly for the treatment of cognitive dysfunctions.
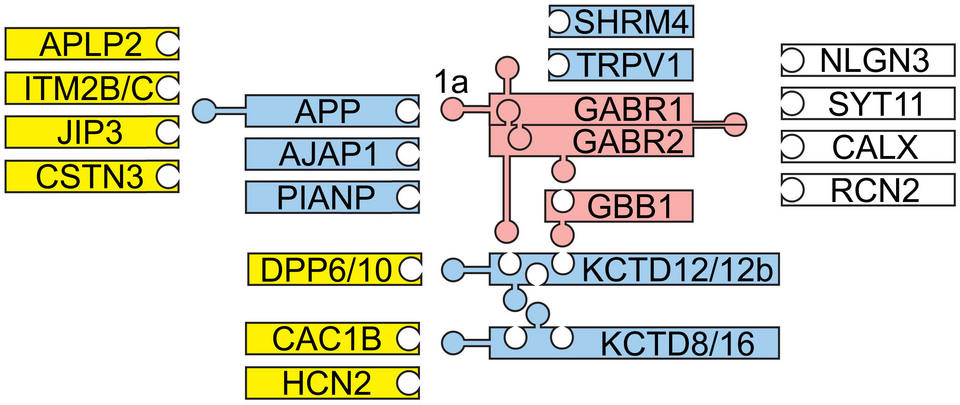
Fig. 1: Structural organization of GBR complexes. GB1 (GABR1) and GB2 (GABR2) receptor subunits constitute fully functional heterodimeric receptors that bind to the β subunit (GBB1) of the G protein (red). Modular association of these receptors with additional components generates multi-protein complexes of varying composition and signaling properties. Primary (blue) and secondary interactors (yellow) as well as receptor components with unknown interaction sites (white) are depicted. Pentamers of auxiliary KCTD proteins bind to GB2 and GBB1 to regulate receptor kinetics. APP, AJAP1 and PIANP bind to the N-terminal sushi domain of GB1a. APP binds to JIP3 and CSTN3, which link the APP/GBR complex to the axonal trafficking motor. APLP2 and ITM2B/C assemble via APP with GB1a. TRPV1 channels bind to GB1a while Cav2.2 (CAC1B) and HCN2 channels bind to KCTD8/16. NLGN3, Neuroligin 3; SYT11, Synaptotagmin 11; CALX, Calnexin; RCN2, Reticulocalbin 2. Adapted from Fritzius & Bettler, Basic Clin. Pharmacol. Toxicol. 126, 2020.
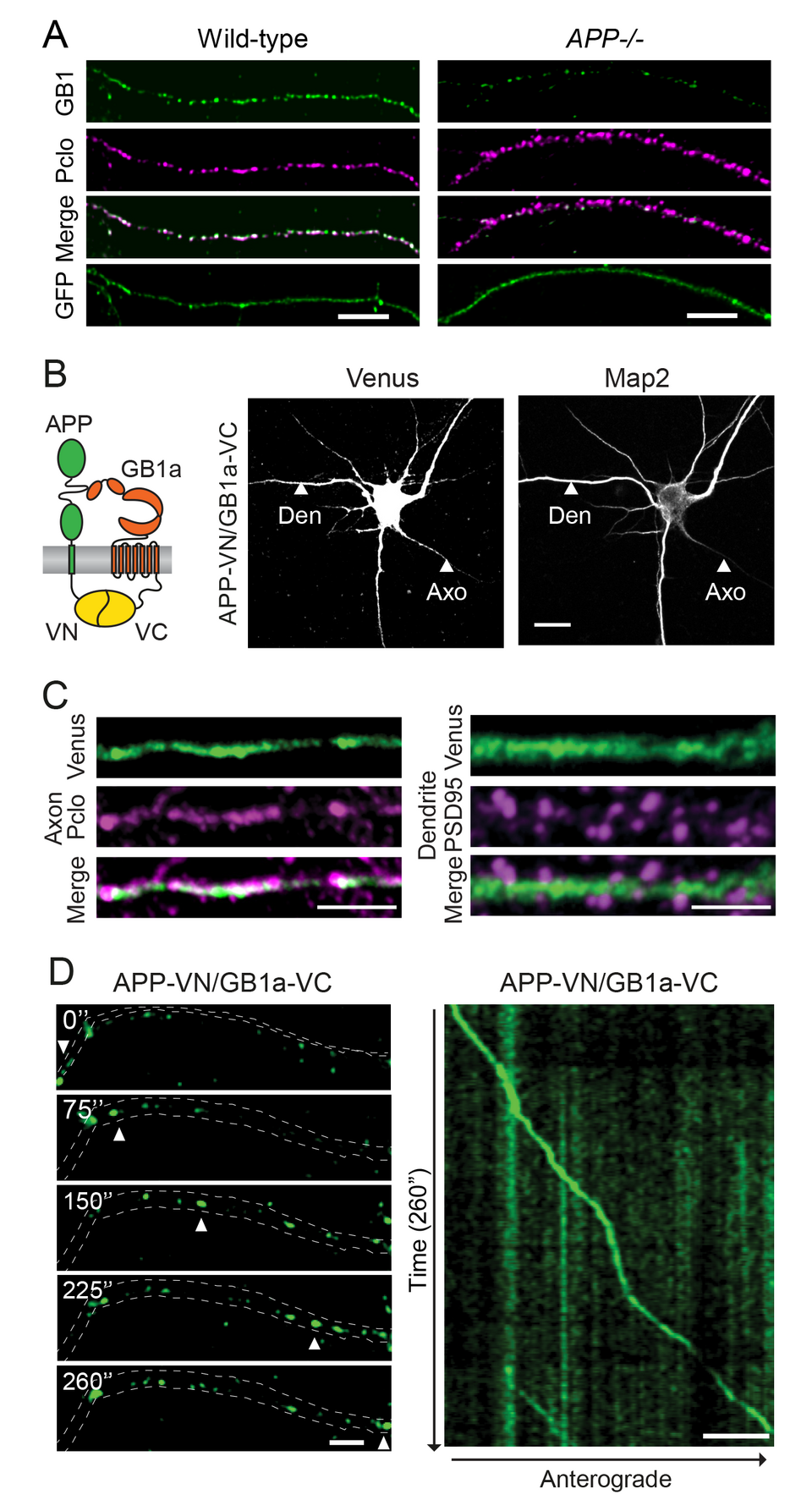
Fig. 2: APP associates with GB1a and mediates axonal trafficking of GBRs. (A) Endogenous GB1 protein is reduced by 75% in the axons of APP-/- hippocampal neurons. Neurons were immunostained for GB1 (green) and the presynaptic marker piccolo (Pclo, magenta). GFP served as a volume marker. Merged images show GB1 and piccolo co-localization. Scale bar 5 μm. (B) Scheme depicting bimolecular fluorescence complementation (BiFC) using the split Venus fusion-proteins APP-VN and GB1a-VC. Association of APP-VN with GB1a-VC reconstitutes Venus fluorescence. Representative confocal images show hippocampal neurons expressing APP-VN together with GB1a-VC. BiFC is observed in axons (Axo) and dendrites (Den). Microtubule-associated protein Map2 identifies dendrites. Scale bar 10 μm. (C) Higher magnification of axons and dendrites expressing APP-VN and GB1a-VC. The BiFC complex (Venus) partly co-localizes with piccolo (magenta) and is also present along dendritic shafts but excluded from spines, identified by PSD-95 (magenta). Scale bar 5 μm. (D) Time-lapse images of a well-separtated APP-VN/GB1a-VC complex (arrowheads) trafficking anterogradely in axons (acquisition time in seconds). A kymograph shows the entire time-lapse recording (right). Scale bars 25 μm.
